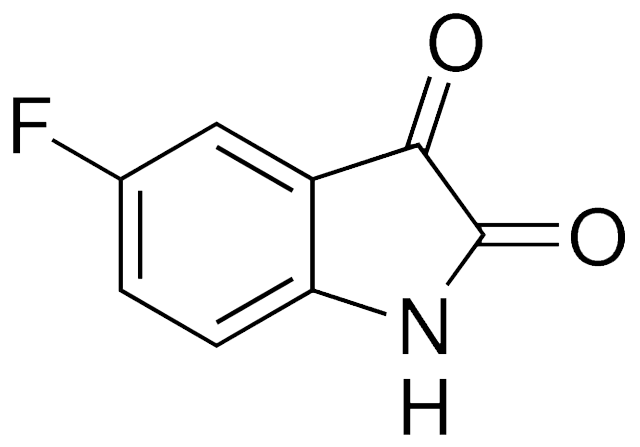
属性
描述
产品介绍
5-Fluoroisatin has been reported as the precursor of the Sunitinib (Sutent) drug. 5-Fluoroisatin has been approved by the Food and Drugs Administration (FDA) in 2006 for the treatment of renal cell carcinoma (RCC) and gastrointestinal stromal tumor (GIST).
别名 5-氟二氢吲哚-2,3-二酮;5-氟吲哚啉-2,3-二酮;5-氟吲哚醌;5-Fluoroindole-2,3-dione;5-Fluoro-2,3-indolinedione
应用 5-Fluoroisatin may be used as reaction-based probe for live-cell detection of peroxynitrite by 19F magnetic resonance spectroscopy;in non-invasive detection of peroxynitrite (ONOO(-)) formation in living lung epithelial cells stimulated with interferon-γ (IFN-γ);in the synthesis of bis-Schiff bases, via condensation with aromatic primary bis-amines in water suspension medium without using any organic solvent or acid catalyst;in the synthesis of 3-acetonyl-5-fluoro-3-hydroxyoxindole
Reactant for preparation of:Spiro[indole-thiazolidinones] as biologically relevan synthesis scaffolds;Potential antimycobacterial agents;Inhibitors of c-Met kinase;Inhibitors of TAK1 kinase;Herpes simplex virus inhibitors;IKKβ inhibitors; Inhibitors of vitiligo disease; Potential drug candidates with anti-HIV activity and anti-tubercular activity
 021-54720761
点我QQ咨询
点我QQ咨询
021-54720761
点我QQ咨询
点我QQ咨询
共有(72)人参考评价
仅对购买过该产品的用户开放!
{comment_time|今天}
{content|默认评论}
{comment_time|今天}
{content|默认好评}
{comment_time|今天}
{content|默认中评}
{comment_time|今天}
{content|默认差评}